
2 Answers
-
asked Sep 30 12:00:00 AM
Sangeeta
Q: 22 A: 1924 -
4 Years Ago
So here the correct answer is "D"
These three types of isomerism are shown in Pentanone
- Chain Isomerism
- Position Isomerism
- Functional Isomerism
Let's discuss how?
First, we should know about what is Isomerism?
Isomerism - Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures.
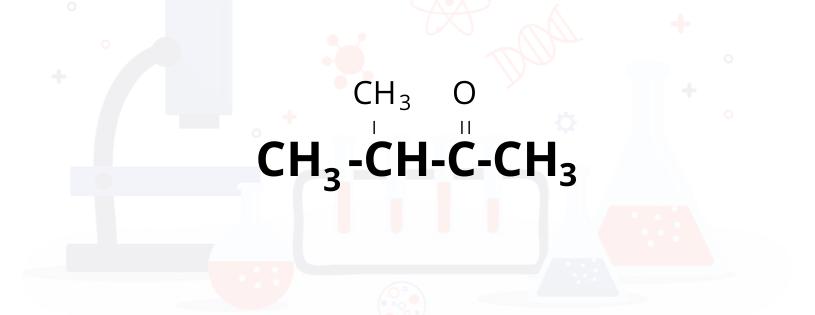
We have given compound pentaone , Below we are making the structure of 5 Carbon chain for pentaone compound
Structure of compound shown below after adding ketonic functional group and balancing
Now we will check for which isomerism is possible here
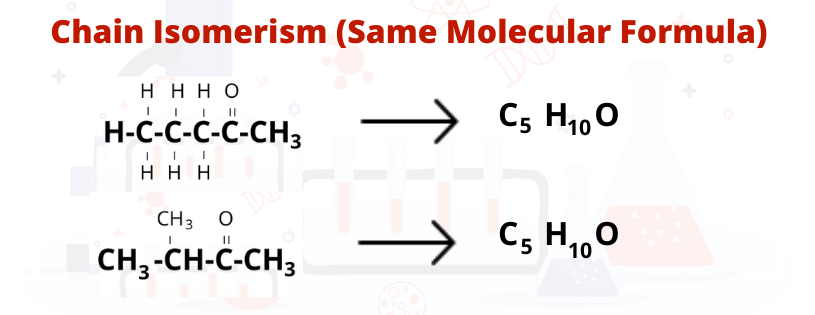
Chain isomerism - First, Here 5 carbon chain possible also a compound available that have 4 & 3 carbon chain and have the same molecular formula, Than structure will be -
Now we will calculate the molecular formula of both figure Isomerism & Chain Isomerism
As shown above, both have the same Molecular formula than Chain Isomerism is possible here.
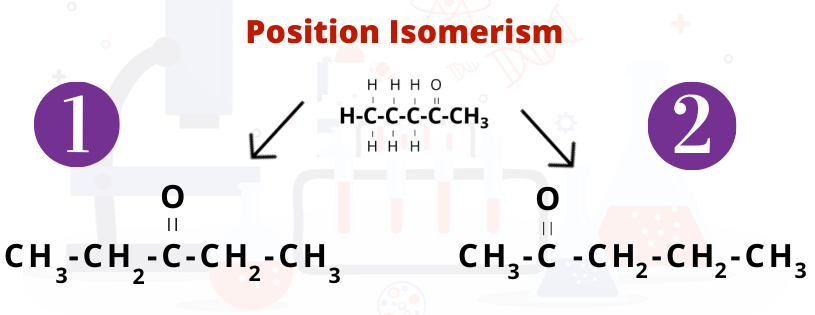
Position Isomerism - Position isomerism, an example of structural isomerism, occurs when a functional group is in different positions on the same carbon chain.
asked Apr 25 12:00:00 AM
Harshit Shrivastav
Q: 617 A: 110




.png)

